Vaccine Injury Statute of Limitations in 2026
You have a limited time to file a vaccine injury claim. Missing the deadline means you cannot recover compensation, no matter how strong your case is.
6 min read
Vaccine Injury Law Resources / Vaccine Court / What is the NCVIA?
 Paul Brazil
:
Nov 10, 2025 10:46:55 AM
Paul Brazil
:
Nov 10, 2025 10:46:55 AM
The National Childhood Vaccine Injury Act is the law that protects vaccine access, funds injury compensation, and sustains the nation’s vaccination system.
The National Childhood Vaccine Injury Act of 1986 (NCVIA) is the cornerstone of vaccine law in the United States. Passing the bill allowed Congress to protect public health, stabilize vaccine costs, and create a system for vaccine injury compensation that works for families and the vaccine manufacturers who supply the nation’s childhood vaccines.
The law established the Vaccine Injury Compensation Program, administered by the Department of Health and Human Services through the Health Resources and Services Administration. This federal program ensures that anyone injured by a covered vaccine, including a vaccine recipient, parent, or legal guardian, can pursue a claim in the Court of Federal Claims.
By tying each award to a trust fund supported by a vaccine excise tax, the NCVIA preserves the vaccine market while maintaining accountability for verified vaccine related injuries. It continues to shape how health care providers report adverse events, how new vaccines are reviewed, and how vaccine injury claims are decided under federal health and human services oversight.
Before 1986, courts were crowded with vaccine injury claims filed under the traditional tort system. Each case required proof of fault against vaccine manufacturers, and verdicts varied widely. Legal fees grew while judgments created uncertainty across the industry. Manufacturers faced mounting liability and insurance costs that threatened to remove essential childhood vaccines from the market entirely.
Congress recognized that public health depended on a stable vaccine market, but injured patients still needed a clear legal path to recover compensation. The National Childhood Vaccine Injury Act of 1986 reframed liability as a federal claim under the Vaccine Injury Compensation Program rather than a civil lawsuit. This shift centralized claims within the U.S. Court of Federal Claims, where each petition is reviewed by a special master under the supervision of the Department of Health and Human Services.
The result was a streamlined process that protected the vaccine market, reduced legal inconsistency, and ensured those with legitimate vaccine caused injuries received payment through a federally funded trust instead of through jury verdicts. It remains one of the most significant legal developments in modern public health law.
Take Control of Your Injury Today
Since 1986, the system created by the NCVIA has paid out more than $5 billion to people who proved they were injured by vaccines. Every case is filed in a federal court dedicated to these claims and reviewed by a special master who decides whether the medical evidence supports compensation.
The process is straightforward but exacting. A claimant submits proof that a covered vaccine caused injury, backed by records from treating doctors. If the case meets federal standards, payment is issued from a national trust fund supported by a small tax on each vaccine dose.
Awards vary depending on the evidence and severity of the injury. Some cover months of physical therapy; others exceed a million dollars for lifelong disability. Typical payouts include medical expenses, time lost from work, and damages for pain and suffering.
For families dealing with verified injuries, this program is often the difference between bearing the cost alone and receiving meaningful compensation for recovery. It’s how the law turns evidence into results.
The National Childhood Vaccine Injury Act outlines how vaccine injury cases are built, filed, and awarded. These provisions define what counts as evidence, what vaccines qualify, and how compensation is funded. For anyone considering a claim, these rules form the foundation of the process.
1. The Vaccine Injury Table
The vaccine injury table is the reference every claim depends on. It lists each covered vaccine, the recognized injuries, and the time window in which symptoms must appear. If your injury matches one of these criteria, it’s presumed to be vaccine caused, which shortens the legal process. For conditions not listed, attorneys must prove a causal link through medical records and expert reports.
2. Reporting and Medical Evidence
Federal law requires healthcare providers to document and report any serious adverse events that occur after vaccination. These reports often serve as key supporting evidence in a claim. When filed correctly, they help establish timelines and strengthen proof of injury. Inconsistent or missing reports can make the special master’s review more challenging.
3. Vaccine Information Requirements
Before vaccination, providers must give patients vaccine information materials outlining the benefits and risks of each immunization. These handouts are part of informed consent. If you were not given this information, it doesn’t disqualify your case, but it adds context to your record and helps your attorney document the interaction.
4. Funding Through the Excise Tax
Every dose of vaccine sold includes a small excise tax that supports the federal trust fund used to pay successful claims. This system ensures that compensation is available without reducing the national vaccine supply or driving up vaccine costs. It’s one of the Act’s most effective financial safeguards and allows payments to remain consistent regardless of the number of claims filed each year.
5. The Role of the Special Master
Each case is assigned to a special master in the U.S. Court of Federal Claims. Their role is to review evidence, assess medical consistency, and determine eligibility for compensation. There are eight in total, and their written decisions guide how future cases are interpreted. Their focus is always on evidence, timing, and reliability.
6. The Advisory Commission on Childhood Vaccines
This independent commission reviews claim trends and recommends updates to the injury table when new scientific evidence emerges. It has influenced the inclusion of new vaccines over time, ensuring that modern immunizations, like the human papillomavirus vaccine, are considered alongside classic childhood vaccines such as measles, mumps, rubella, and pertussis.
The National Childhood Vaccine Injury Act created opportunity, but it also created obligation. Every petition filed under this law must meet exact federal standards for timing, evidence, and documentation. Missing even one can limit or delay compensation.
A claim begins with strict deadlines. Most must be filed within three years of the first symptom, and the petition must include precise details about the vaccine type, administration date, and medical findings. The Court of Federal Claims will not review incomplete filings.
Evidence demands are equally high. Petitioners must present verified medical records, detailed diagnostic results, and expert statements that link the injury to a covered vaccine. The special master reviewing the case expects this proof to meet the legal definition of causation, not speculation, not assumption, but substantiated medical reasoning.
Only attorneys admitted to practice in this federal court can submit and argue these claims. They must understand how the vaccine injury table interacts with precedent, how to calculate awards under program guidelines, and how to respond to government counsel. Few lawyers have that experience.
At My Vaccine Lawyer, we handle these cases every day. We know how to assemble the evidence, interpret the deadlines, and prepare the petition so it stands up to scrutiny. Each claim is structured to meet the court’s expectations the first time, avoiding the setbacks that cost claimants time and money.
If you believe you’ve suffered a vaccine caused injury, the next step is legal precision, not guesswork. Call us on (800) 229-7704 or fill out a quick form and we’ll tell you exactly what’s required and how to move your claim forward.
The Vaccine Injury Compensation Program (VICP) was created under the Injury Act of 1986, also known as the National Childhood Vaccine Injury Act. It offers a no-fault route for vaccine injury compensation, allowing people injured by vaccines covered in the program to recover financial awards. The Act balances individual recovery with public health needs by protecting the national vaccine supply while ensuring victims of adverse events can seek compensation.
Anyone who received a covered vaccine after October 1, 1988 may file a claim through an attorney. Lawyers also file on behalf of children, disabled adults, or families of deceased individuals. The case must show a reasonable basis for injury and be filed in good faith to qualify for compensation. Even other cases outside the listed categories may be considered if strong medical evidence shows the vaccine caused disease or harm.
The National Vaccine Injury Compensation Program applies to certain vaccines recommended by the CDC, including measles, mumps, rubella, pertussis, influenza, and human papillomavirus. Such vaccines are routinely given to prevent serious disease and are reviewed regularly as medical research evolves. The list of vaccines covered, known as the vaccine injury table, is updated when new scientific data becomes available.
If a doctor or clinic administers vaccines that are not yet listed as covered, claims may still move forward if scientific research later supports a causal link. Attorneys can petition to have new vaccines added, and the special master will review the evidence alongside data from adverse event reporting systems. These cases often help expand the list of certain vaccines eligible for compensation.
Federal law requires healthcare providers to submit adverse event reporting when a patient experiences a reaction after vaccination. These reports are stored in a national database monitored by the CDC and are often used as evidence in vaccine injury claims. Consistent reporting helps attorneys establish medical timelines, verify patterns of reaction, and strengthen the link between vaccination and injury.
Unlike broader public health statutes, the NCVIA directly governs legal recovery for vaccine injuries. It defines who may file, how compensation is calculated, and how claims are reviewed. Each award under this act reinforces the same goal: protecting individuals harmed by vaccines while preserving the overall strength of the vaccination system.
Take Control of Your Injury Today
Paul Brazil is a native of Dunmore, Pennsylvania and a graduate of Dunmore High School. For his undergraduate education, he attended Bloomsburg University where he majored in political science. He then went on to earn his JD from Widener University School of Law. Following graduation from law school, Mr. Brazil worked at a large Philadelphia civil defense firm where he litigated workers’ compensation claims and Heart and Lung Act cases. In 2012, he joined with his coworker Max Muller to form Muller Brazil.

You have a limited time to file a vaccine injury claim. Missing the deadline means you cannot recover compensation, no matter how strong your case is.

If you're feeling arm pain after a vaccine, it's common and usually not serious, often caused by your body's reaction to the vaccine which causes...
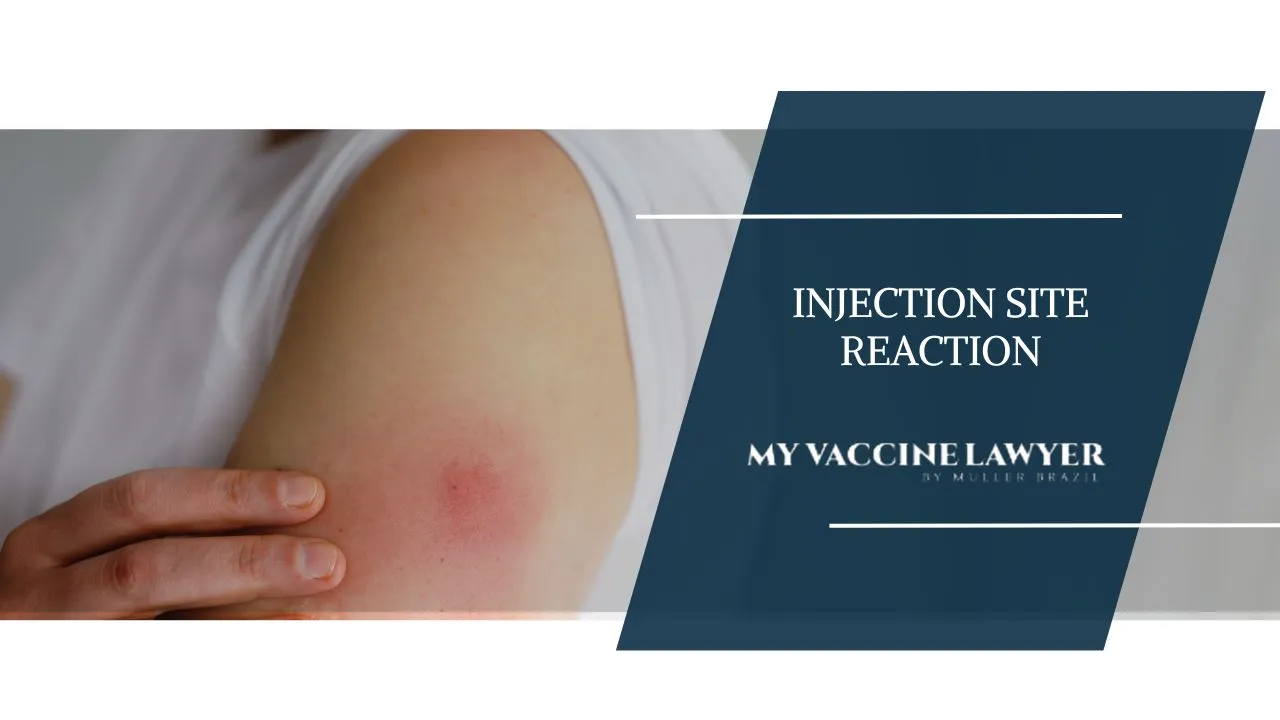
In this blog post, we will dive into the various types of vaccine injection site reactions, their symptoms, treatment and how to prevent and manage...
.png)