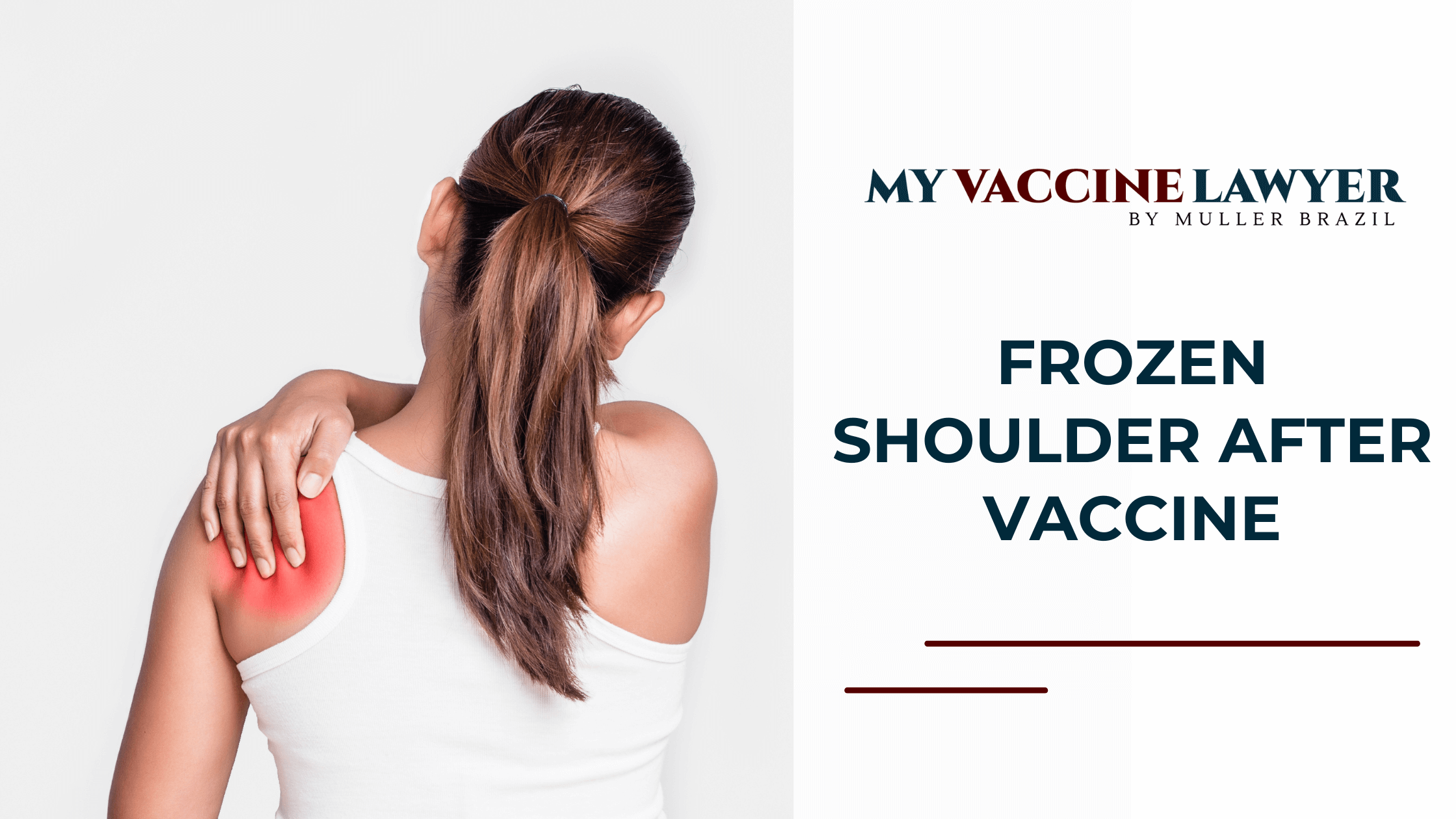
Frozen Shoulder After Vaccine
Frozen shoulder after vaccine claims are rising, and so are settlement values. Clients presenting with painful shoulder stiffness, reduced range of...
7 min read
Vaccine Injury Law Resources / Pneumococcal / Understanding Pneumococcal Vaccines and Vaccine Injury Compensation
 Max Muller
:
Sep 20, 2023 9:18:59 AM
Max Muller
:
Sep 20, 2023 9:18:59 AM
Pneumococcal vaccines are given every day in clinics, pharmacies, and hospitals. When the outcome changes, the legal system treats those cases very differently.
Pneumococcal vaccines are still part of routine immunization practices across multiple age groups in the United States in 2026. They are administered to reduce the risk of serious bacterial infections caused by streptococcus pneumoniae, including pneumonia, meningitis, and bloodstream involvement. Public health guidance highlights their role in disease control and prevention, particularly for young children, older adults, and people with chronic heart disease, chronic lung disease, or other underlying medical conditions.
What is rarely addressed is how these vaccines appear inside the Vaccine Injury Compensation Program (VICP) when adverse outcomes occur. Claims linked to pneumococcal conjugate vaccine and pneumococcal polysaccharide vaccine administration include shoulder injuries related to injection technique (SIRVA), neurological conditions involving the spinal cord, severe allergic reaction, and febrile seizures in children younger than five years of age. In a number of settlements, symptoms developed after the expected immune response window, which delayed recognition that the vaccination itself may have been legally relevant.
Pneumococcal vaccines are not a single product. In clinical practice and in the Vaccine Injury Compensation Program, the type of vaccine given often shapes how an injury is evaluated and why certain claims follow familiar patterns. That distinction rarely matters at the appointment itself. It becomes relevant only when symptoms persist or escalate beyond the expected immune response.
Two categories are used in routine pneumococcal vaccination. Each shows up differently in injury claims.
(PCV13, PCV15, PCV20)
These vaccines are commonly administered to young children and older adults during scheduled care, often alongside other immunizations. They trigger a targeted immune system response against specific pneumococcal serotypes associated with serious disease such as pneumococcal pneumonia and invasive pneumococcal disease.
Medical records in compensation claims following conjugate vaccination have documented conditions including:
(PPSV23)
This vaccine is more commonly administered to adults years of age with increased risk factors, including chronic heart disease, chronic lung disease, sickle cell disease, cochlear implants, or cerebrospinal fluid leaks. It is often given as a single dose during visits focused on long term disease control rather than routine childhood immunization.
Claims involving PPSV23 often involve adults with existing health conditions, which can complicate early attribution. Diagnoses documented in medical records have included:
Claims evaluated through the Vaccine Injury Compensation Program include documented outcomes such as persistent shoulder injury related to injection technique, neurological conditions involving the spinal cord, severe allergic reaction requiring emergency treatment, and febrile seizures in children younger than five years of age. In a number of settlements, the injury was not identified at the time of vaccination and became legally relevant only after symptoms failed to resolve.
The type of vaccine given is rarely questioned during administration. It becomes significant later, when recovery does not follow the expected course and the legal evaluation turns on timing, symptom progression, and documented medical response. That distinction sets the foundation for separating common side effects from injuries that qualify for compensation.
A diagnosis does not create a claim on its own. It changes how the medical record is read. Once a condition is identified and documented as persistent or progressive, the sequence between vaccination, symptom onset, and treatment becomes legally reviewable. That is the point at which compensation analysis begins.
Every vaccination produces an immune response. That response is expected, temporary, and closely monitored in clinical settings. In vaccine injury claims, the issue is not whether symptoms appeared but whether they resolved within the normal recovery window or progressed into a condition that limited function, required treatment, or altered daily life. That distinction guides how pneumococcal vaccination cases are evaluated inside the Vaccine Injury Compensation Program.
These effects are widely documented and typically improve without medical intervention:
These reactions are anticipated and are not treated as compensable injuries.
Claims involving pneumococcal vaccines reflect a different trajectory, one marked by persistence, escalation, or functional loss:
These patterns appear repeatedly in settlement records and form the basis for legal evaluation.
Several factors delay recognition in vaccine injury cases. Early symptoms are frequently attributed to age, chronic heart disease, chronic lung disease, or other underlying medical conditions. Initial guidance often emphasizes waiting for improvement rather than documenting progression. Imaging, specialist referral, or neurological evaluation may occur only after symptoms persist, at which point the vaccination itself is rarely revisited without legal review.
In compensation claims, the difference between a side effect and a recognized injury is established through timing, documentation, and impact on daily function. That analysis determines whether a claim proceeds and why many cases begin only after recovery fails to follow the expected course.
Compensation awarded through the Vaccine Injury Compensation Program is tied to documented costs and measurable loss. In pneumococcal vaccine claims that meet the legal threshold, awards commonly fall into five-figure to six-figure ranges, depending on how long treatment continued and whether full recovery occurred. Pain and suffering damages are capped at $250,000 under federal law. Medical expenses and lost income are not capped.
Rather than abstract categories, compensation has reflected itemized care recorded in medical files, including:
These figures appear in claims where symptoms did not resolve within the expected post vaccination period.
In cases where recovery stabilized without full resolution, compensation has addressed anticipated care needs documented before claim resolution, including:
Future care is awarded when providers document necessity rather than possibility.
Loss of income is evaluated using records already in existence. In pneumococcal vaccine claims, compensation has included:
For self employed individuals, tax records and business income statements are used rather than estimates.
Pneumococcal vaccine injury claims must generally be filed within three years from the first symptom of injury. Once filed, claims commonly remain active for two to four years, depending on medical complexity and expert review. Attorney fees and expert costs are paid by the program when claims meet filing requirements and are not deducted from the award.
Award size is driven by documentation depth rather than diagnosis labels. Claims supported by the following consistently resolve at higher values than claims supported by short term or fragmented care:
See what other victims of vaccine injury claims have won when partnering with My Vaccine Lawyer.
Pneumococcal vaccine injury claims are rarely dismissed because the injury is insignificant. They are missed because the way these injuries develop does not align with how post-vaccination follow-up typically works. In many cases, the problem is not lack of harm but lack of early recognition and documentation.
Age and existing health conditions
A large percentage of pneumococcal vaccinations are given to older adults or individuals with chronic heart disease, chronic lung disease, or other underlying medical conditions. When symptoms appear, they are often attributed to aging, preexisting illness, or general decline. That assumption delays investigation into whether the vaccination played a role, even when symptoms follow a clear change in physical function.
“Wait and see” medical guidance
Early follow-up frequently consists of reassurance rather than documentation. Patients are told soreness, weakness, or fatigue should resolve on its own. When improvement does not occur, weeks or months may pass before imaging, referral, or specialist evaluation is ordered. By then, the vaccination is no longer part of the clinical discussion, even though the timeline remains relevant under the Vaccine Injury Compensation Program.
Delayed escalation of symptoms
In pneumococcal vaccine claims, symptoms do not always peak immediately. Loss of shoulder mobility, neurological changes, or functional limitation may progress gradually after the expected immune response period. This pattern does not match the typical expectation of short-term post-vaccination effects, which contributes to delayed recognition rather than early dismissal.
Lack of awareness of federal compensation programs
Most healthcare providers do not discuss the Vaccine Injury Compensation Program with patients. As a result, individuals often assume there is no legal pathway available once symptoms persist. Claims are frequently identified only after independent research, long after medical care has already been underway.
A claim does not fail because recognition was delayed. It fails only when documentation does not show persistence, progression, or impact. Delayed filing is common in pneumococcal vaccine cases and does not prevent compensation when the record supports it.
If you are researching pneumococcal vaccines because recovery did not follow the expected course, timing matters. The Vaccine Injury Compensation Program applies strict filing rules based on symptom onset, not diagnosis or referral.
My Vaccine Injury Lawyer focuses exclusively on vaccine injury claims handled through the federal program. Our mission is to bear the weight of the Vaccine Injury Compensation Program process so you can stay focused on your and your family's health. While being confident you're building the best case for compensation.
When you work with us, you get more than legal representation by the nation's premier vaccine injury law firm. You get peace of mind knowing every step of the legal process is being handled effectively. Our vaccine injury lawyers simplify the vaccine injury legal process, at no cost to you.
Request a free consult or call (800) 229-7704
Mr. Muller currently devotes the majority of his law practice to aggressively fighting for the victims of unsafe drug and medical device injuries, as well as vaccine injuries and vaccine reactions involving the flu shot, TDaP/DTaP vaccine, and more. He has handled hundreds of SIRVA injury cases (shoulder injury related to vaccine administration), especially those involving bursitis, tendonitis, frozen shoulder, and rotator cuff tears. Mr. Muller also handles cases where vaccines caused serious nerve injuries such as Guillain-Barre Syndrome. Mr. Muller has recovered millions of dollars in compensation for his clients in the Vaccine Injury Compensation Program.
Frozen shoulder after vaccine claims are rising, and so are settlement values. Clients presenting with painful shoulder stiffness, reduced range of...

Attorney Max Muller was retained by a woman who suffered a shoulder injury following a flu shot and pneumonia vaccine. Immediately following the...

To prevent arm soreness after receiving a vaccine, make sure to hydrate well and get good rest before your appointment. Avoid alcohol and caffeine as...
